श्री चित्रा तिरुनाल आयुर्विज्ञान और प्रौद्योगिकी संस्थान, त्रिवेंद्रम
विज्ञान एवं प्रौद्योगिकी विभाग, भारत सरकार के अधीन एक राष्ट्रीय महत्व का संस्थान
Sree Chitra Tirunal Institute for Medical Sciences and Technology, Trivandrum
An Institution of National Importance, Department of Science and Technology, Govt. of India






श्री चित्रा तिरुनाल आयुर्विज्ञान और प्रौद्योगिकी संस्थान, त्रिवेंद्रम
Sree Chitra Tirunal Institute for Medical Sciences and Technology, Trivandrum
- Home
-
About Us
-
- STATUTORY & IMPORTANT COMMITTEES
- Institutional Ethics Committee
- Institutional Biosafety Committee
- Institutional Animal Ethics Committee
- Public Grievance Committee
- more..
- Services
-
Portals
-
- PATIENT PORTAL
- Help
- Visit Portal
-
- PENSIONERS PORTAL
- Help
- Visit Portal
-
- VENDORS PORTAL
- Help
- Visit Portal
-
- Customer Service Portal
- Visit Portal
- Student Portal
- Visit Portal
-
- BLOOD DONORS PORTAL
- Visit Portal
- Smrithi Vanam Portal
- Visit Portal
-
- TIMED
- Visit Portal
- Alumni
- Visit Alumni Website
-
-
News and Resources
-
- Conferences/Events
- Upcoming Conferences/Events
- Previous Conferences/Events
- Academic & Research
- Intellectual Property Rights, Patents
- Academic Programmes
-
Recruitment
-
- Job Opportunities
- Active Notifications
- Recruitment Conducted
- Archive
-
- Rank Lists
- Latest Rank Lists
- Archive
-
- Previous Question Papers
- Previous Question Papers
- Contact Us
-
Important Links
-
- @SCTIMST
- NIRF
- GATI-SCTIMST
- Happenings
- NIRF
- Donation
Search





Welcome To SCTIMST
Sree Chitra Tirunal Institute for Medical Sciences and Technology (SCTIMST) is an Institution of National Importance under the Department of Science and Technology, Govt. of India. The joint culture of medicine and technology pioneered by its founders more than three decades ago, has come of age and gained unprecedented acceptance in India. The institute has the status of a university and offers excellent research and training facilities. It has three wings: a tertiary referral super specialty hospital, a biomedical technology wing and the Achutha Menon Centre for Health Science Studies.
The Institute focuses on high quality, advanced treatment of cardiac and neurological disorders, indigenous development of technologies for biomedical devices and materials and public health training and research. The institute offers advanced treatment using modern technologies in several specialized areas such as interventional radiology, cardiac electrophysiology, deep brain stimulation for movement disorders, epilepsy surgery, pediatric cardiac surgery, base of skull and vascular surgeries, to name a few. The institute has excellent facilities and teams of professionals dedicated to the development of innovative biomedical devices and products, evaluation of medical devices to global specifications, training in novel medical specialties and research in medical and public health areas of social relevance. The Institute is a Technical Research Centre for Biomedical devices and has a medical devices incubator (TIMed).
Services

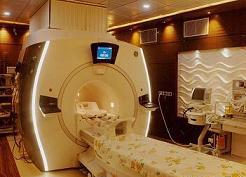
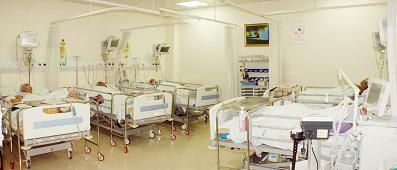

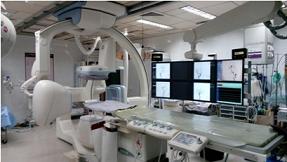


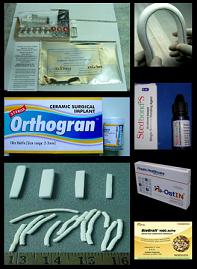








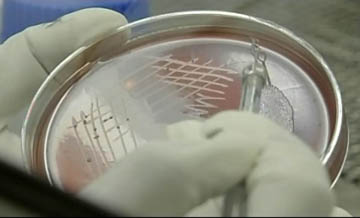


SCTIMST offers post-doctoral courses in Cardiology, Cardiovascular and thoracic surgery, Neurology, Neurosurgery, Cardiac and neuro anesthesia (read more about Academic...)
SCTIMST is focusing on research and development of biomaterials and biomedical devices especially in the areas of cardiac and neuro devices, hard tissue implants(read more about Research...)




Patient Portal
Portal for patients - Providing lab reports, E-Consultations, Prescriptions, Appointment changes etc
Vendors Portal
Portal for Suppliers/Customers to register their bank details for e-payment and for viewing the payment details.


The Institute pursues product development on various category of devices like cardiovascular, neuro prosthetic, hard tissue devices, biological products, In Vitro Diagnostics, Orthotics etc.
Collaborative Programs
The Institute associates with the Industry/academia in programmes/product development of mutual interest. The Institute always entertains product development identified by the industry and take up collaborative development. Please contact tbd@sctimst.ac.in for the same.

The Institute Invites Expression of Interest from interested industry to associate with the process of translation. Kindly contact tbd@sctimst.ac.in for any information on Technology Transfer.
Technology Transfer Policy
The industry may kindly read through the Technology Transfer Policy of the Institute.

Thiruvananthapuram - 695 011, Kerala, India.
Email :sct@sctimst.ac.in
Phone : 91-471-2443152 Fax : 91-471-2446433
PAN NO: AAAJS0437M
TAN : TVDS00986G
GSTIN NO: 32AAAJS0437M1Z4 Date : 28-06-2017








